Lublin Info Centre
Innovative diabetes care: Lublin-based Walletmed’s technology and future plans

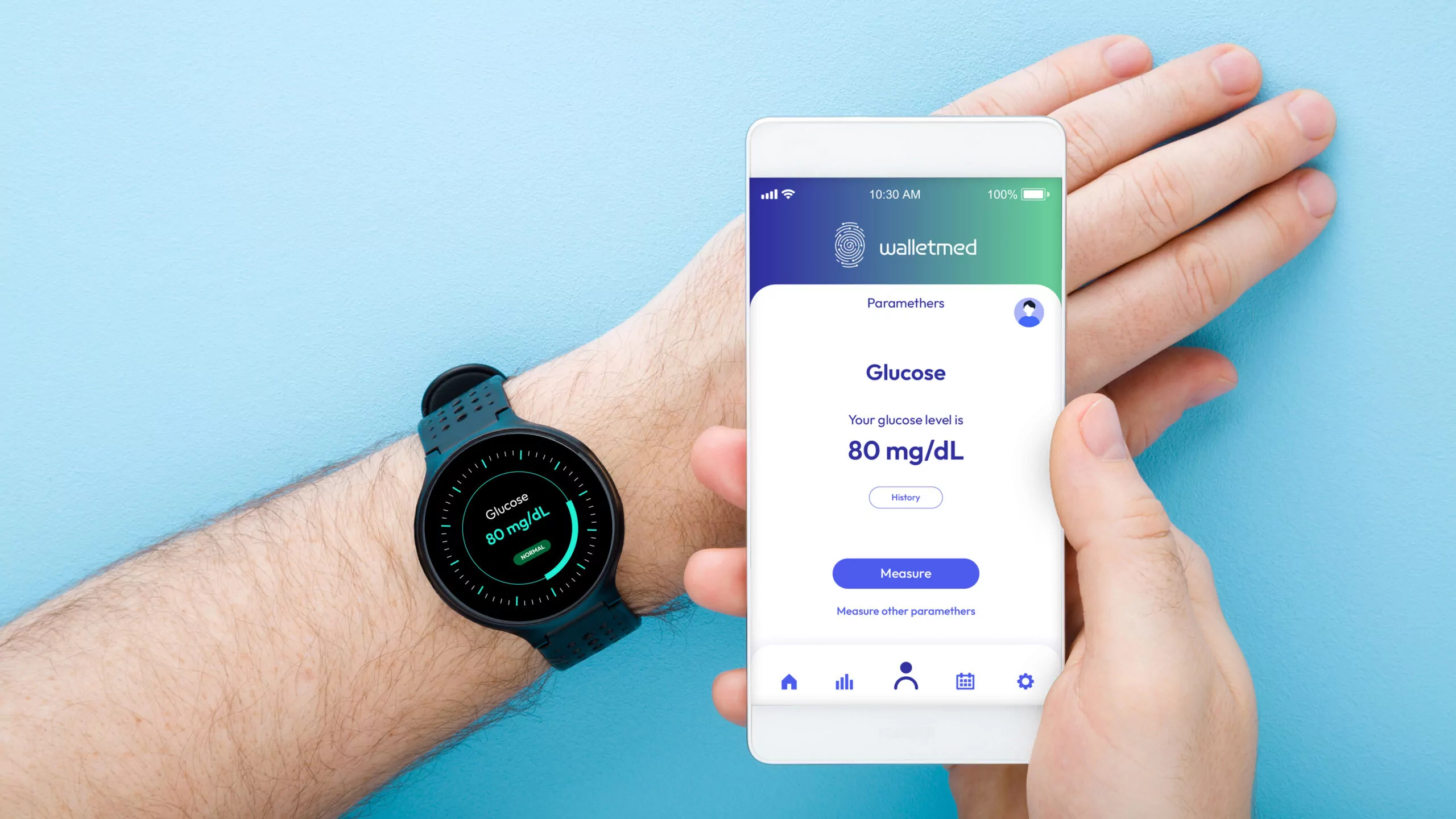
Where did the idea for Walletmed come from, and what exactly is the technology behind the subcutaneous optical implant?
Our first project was Walletmor, the world’s first implant for contactless payments. After several years of working on this project, we realized it was innovative enough to address much more significant problems, beyond the convenience and security of contactless payments, such as monitoring specific substances in our bodies. This led to the creation of our new project, Walletmed. It’s a natural evolution of our product, which can now address even more critical issues.
Current glucose monitoring technologies are quite problematic, as confirmed by surveys we conducted among people living with diabetes. All our respondents said they find finger pricking or changing sensors more inconvenient than a one-time implantation. Additionally, there are issues with the accuracy of glucose measurements and the proper way to take them. With technology like a smart implant that allows for various optical sensors, we can solve these problems and make smart implants even more useful to consumers.
Why did you choose an implant instead of a wearable device? What are the benefits of this solution from both the patient’s and the doctor’s perspectives?
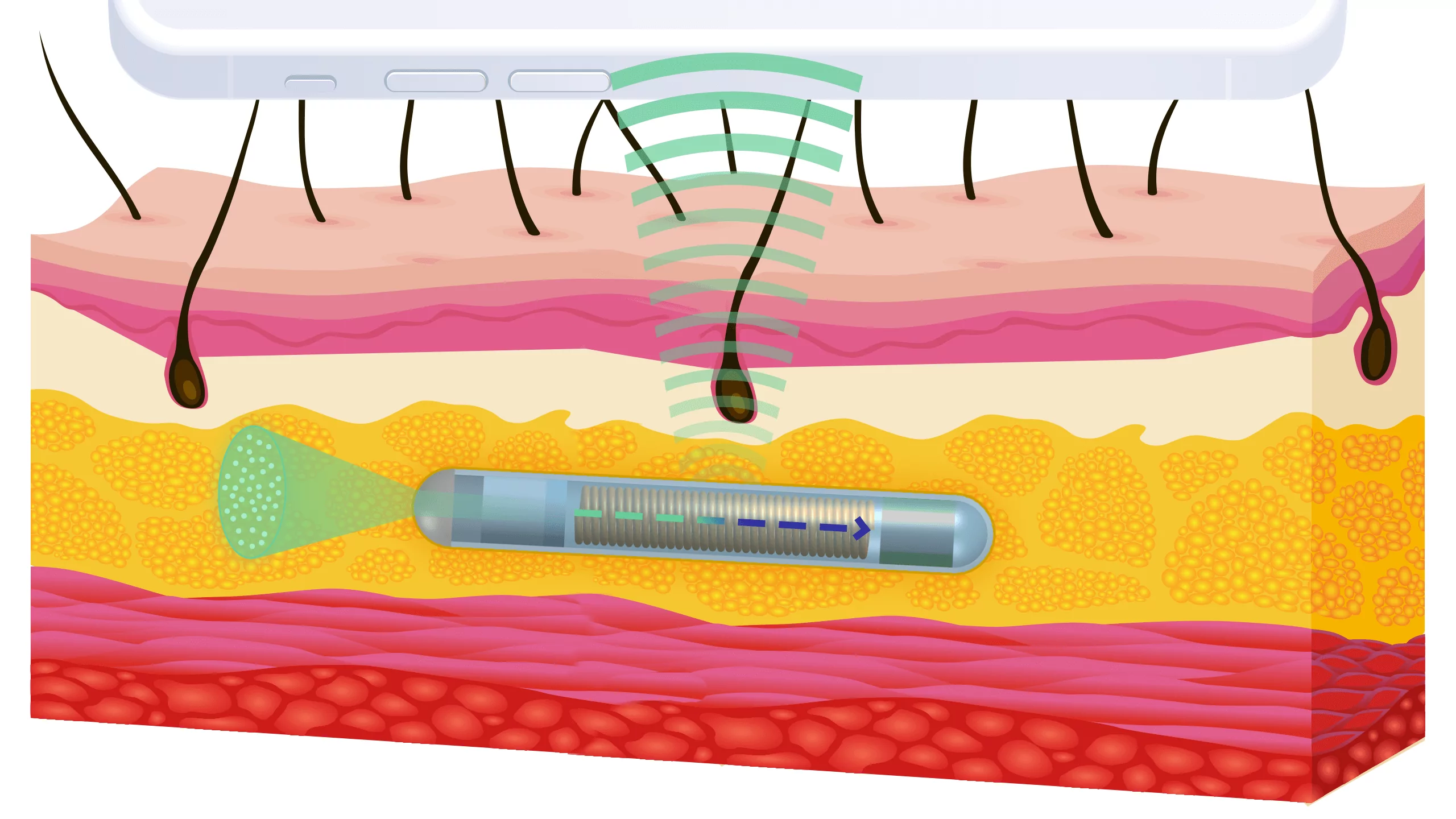
Many major players in the market, as well as startups, have been working for years to create a device that measures glucose or other parameters using wearables. However, from our observations, even after decades of research and development, a smartwatch that is certified as a medical device, providing accurate readings, has not yet been created. In fact, initial R&D efforts began in the 1980s, over four decades ago. Of course, the term “smartwatch” wasn’t in use then, but there were attempts to use laser technologies. Currently, you can buy wearable devices from various online stores whose manufacturers claim they can measure internal body parameters. Unfortunately, none of these devices are certified medical products today. This is due to the physical barrier posed by the skin. In spectrometry-based technologies, the laser has to pass through the skin, take measurements, and return to a detector in the wearable device.

Our research, conducted with medical technology scientists, shows that for each person, regardless of skin type (color, thickness, hair, perspiration, etc.), all these factors must be aligned to get the most accurate readings. We are therefore faced with a choice: either a wearable device that isn’t very accurate and makes it difficult to make serious diagnoses, or a subcutaneous implant that provides the most reliable measurement results.

Speaking of medical device certification, is your product currently in the clinical trial phase?
We are still developing the device, and we have prototypes that we tested on pigs. These tests yielded positive results and confirmed our assumptions. The solution is still in the preclinical stage, but we believe that the results of these studies will serve as a basis for certifying our device as a medical product in both Europe and the United States.
Beyond just providing glucose readings, does your technology offer patients additional capabilities, such as predicting glucose trends or providing personalized therapy recommendations?
Because we use optical measurement technology, we can see a “fingerprint” of biological substances or parameters that have a unique chemical structure. This allows us to measure any organic compound using one device, as the spectrum we obtain is vast and enables a wide variety of measurements important to our bodies. This gives us a significant competitive advantage over other solutions that typically focus on just one method based on either chemical or biological techniques. Our technology, due to its purely physical measurements, can extend to cover all these parameters.
As for prediction capabilities, this is something we certainly plan to implement. Current technology for trend prediction using artificial intelligence is already quite advanced. Having constant access to continuous data and measuring glucose levels every minute allows us to predict trends and, for example, forecast what glucose levels will be after a meal. When we experience hyperglycemia or hypoglycemia, the body naturally alerts us with symptoms like nausea, drowsiness, or other indicators. The goal is to predict this before it happens and react accordingly. We aim for our solution to be a closed-loop system, informing the patient of what might happen and what preventive actions they can take in the coming hours, days, or even weeks.
Are there any similar solutions to your implant on the market?
At the moment, no such solution exists, and we are not aware of any similar technology. We currently have three patent applications pending that protect our intellectual property for this type of project, and we hope to remain a market leader in this technology for a long time. There are many similar solutions on the market, but none utilize flexible diffuse spectrometry technology as we do.
How do you assess the demand for this type of product? Are diabetic patients ready for such a revolution in glucose monitoring?
Our previous experience with Walletmor is very important to us in this regard. We found that while it’s not a life-saving or health-related device, consumers’ approach tends to be more skeptical. We realized through working with our customers, business partners, and potential investors that, as long as a product doesn’t directly impact health or life, people are less inclined to adopt it. The exceptions are technology enthusiasts. However, when we look at cases like pacemakers, cochlear implants, or other medical devices that affect quality of life, or sometimes even life itself, skepticism fades.
There is currently an implant on the market that allows for glucose measurement, but it’s based on chemical glucose monitoring, requiring replacement every three months through a surgical procedure. Our implant is designed to last a lifetime, requiring only a one-time installation. This is a much smaller inconvenience compared to the daily finger-pricking or applying sensors. As I mentioned, we conducted surveys and studies to prove that for diabetic patients, a one-time implantation is less burdensome than the weekly or daily routines of finger-pricking or sensor replacement.
What does your collaboration look like in this specific project with the medical and scientific community? Are you working with specialists from Lublin?
Our product will be a certified medical device, meaning it can be prescribed by doctors just like prescription medications. We are trying to develop as much cooperation and partnership as possible. We have already signed several letters of intent with entities both in Poland and across Europe, expressing interest in distribution and market education. As we get closer to launching the product, we will certainly intensify this cooperation.
An important milestone in the project was conducting preclinical tests of our prototype in collaboration with the Polish Academy of Sciences (PAN) at the Institute of Animal Physiology and Nutrition in Jabłonna. These were professional studies that, of course, had previously obtained the approval of the local ethics committee. The tests were conducted with the utmost care for the well-being of the animals, in accordance with procedures that exceed current bioethical standards. We highly value our cooperation with this institution. This was the first stage of our research, but we have further studies planned.
In Lublin, we have a partnership with CM Luxmed Lublin. It started when Dr. Andrzej Miturski, a co-owner of CM Luxmed, became interested in our solution from a purely medical perspective. He saw the great potential of the product and eventually became one of the first investors and co-founders of Walletmed. He is a co-author of Walletmed’s patent applications to the European and U.S. Patent Offices for the implantable device and metabolite monitoring system. Dr. Miturski is also professionally associated with the 1st Military Clinical Hospital in Lublin and the Medical University of Lublin, where he works as an assistant in the Department of Obstetrics and Pregnancy Pathology. Additionally, one of our advisors is Maciej Maniecki, CEO of Medical Inventi S.A., who provides professional support to the project, particularly as an expert in CE-marked medical devices.
Beyond the Lublin region, we collaborate with Raman spectrometry specialists (the technique we use in our solution) from the Poznań University of Technology and the Institute of Physical Chemistry of PAN in Warsaw.
Are you considering expanding into international markets? If so, which international markets do you consider the most promising for your products and why?
Of course, we aim to expand internationally, so the EU and U.S. markets are definitely our primary targets. These are natural directions for product distribution after certification, given the size of these markets and their patient maturity. Let’s not forget that the CE mark allows for sale across the entire EU. In the U.S., if someone already has insurance for the reimbursement of medical devices related to glucose monitoring, which is necessary for diabetics, the cost is automatically covered by the insurer. Additionally, the U.S. has a large population of diabetics, and we’re witnessing this growing negative trend. Fortunately, there is an increasing number of people who are aware and want to manage their glucose levels for their comfort.
What are the company’s future development plans? Can Walletmed’s technology be used in other areas of medicine beyond diabetes care?
Our comprehensive product, which consists of an implanted sensor, wearable device, and application, will need to undergo the medical device certification process. Our biggest challenge at the moment is focusing on one crucial aspect – obtaining the most accurate data possible, which will allow us to navigate clinical trials successfully and achieve product certification. We are working on this and believe that we will soon be ready to launch our product on the market.
In response to the second part of your question, our plans certainly include expanding the technology, which is capable of measuring various parameters in the human body, not only glucose but also insulin, cortisol, cholesterol, etc., which influence various metabolic diseases. The method we use to measure specific metabolites has broad applications, particularly in the field of perinatal care. As is well known, the bodies of pregnant women are particularly sensitive to changes in various metabolites. Therefore, expanding our technology into other areas of medicine has enormous potential. Measuring additional parameters beyond glucose, combined with analyzing their interrelationships, can provide significant diagnostic information for various patient groups.
It is also worth emphasizing that the technology we are developing at Walletmed has wide-ranging applications in fields beyond medicine. Our solution can also be used in the pharmaceutical industry (measuring active substances in the body during clinical trials and monitoring side effects), veterinary medicine (monitoring vital functions in animals), military applications (monitoring soldiers’ vital signs), quality control (e.g., food products), and even chemical analysis (detecting and measuring microplastics and other contaminants in liquids).
Thank you for the conversation.






