Lublin Info Centre
ADHI – Leading innovations in cardiology and prosthetics from Lublin

What led you to focus on medical technologies and prosthetics as key areas for business development?
I have always been fascinated by technology, which led me to apply for the newly established Biomedical Engineering program at the Lublin University of Technology. By my second year, I had started working for a Warsaw-based company producing medical devices, including coronary stents. The engineer who developed these stents, Łukasz Wasyluk, is now part of my team. During my fourth year, the company established a research and development facility in Puławy for me to manage, which helped me balance work with my education. Working in such a company allowed me to understand the market dynamics, the needs of customers, and to build connections within the medical field. Thanks to the involvement of the medical community and the expertise of the specialists and practitioners I met during my career, we developed five clinically needed technologies, three of which are intended for interventional medicine and two for prosthetics:
– **Acti Stent**: An esophageal stent for cancer patients.
– **Clandev**: A CAD device for removing thrombi from cerebral venous sinuses.
– **Opti G**: An advanced diagnostic guidewire for coronary arteries that allows for embolic structure assessment.
– **Exomedis**: An advanced prosthetic socket manufacturing technology that automates production and creates universal sockets available in various sizes.
– **NK TECH**: Innovative prosthetic sockets that can adjust their volume to the patient’s stump in real time.

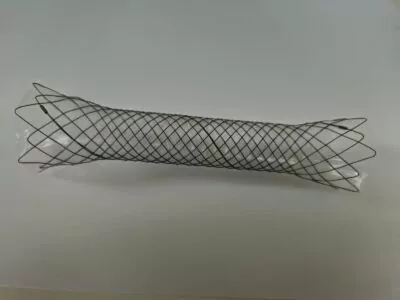

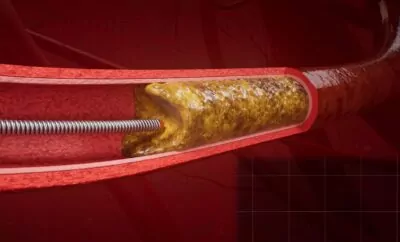



You graduated from the Lublin University of Technology, which is well-known for its strong track record in patent filings and inventions. What does this mean for business?
Owning patents facilitates the transfer of technology to the industry, which is crucial for improving existing products or creating new, innovative solutions. One of our companies, NK-Tech, is a spin-off that emerged from the Lublin University of Technology and is a great example of successful knowledge transfer from academia to the market.
Together with Dr. hab. inż. Tomasz Klepka, professor at the university and head of the Technology and Polymer Processing Department, we sought new technological and material solutions to improve the quality of life for adults and children with limb amputations. We worked in an interdisciplinary team of experts in biomaterials, biomedical engineering, and limb prosthetics. We developed a new-generation silicone prosthetic socket because existing solutions were often uncomfortable and expensive. Our innovative solution uses high-quality materials like HTV medical silicone and features a functional design that adapts to the stump’s volume. We created a pneumatic system allowing patients to adjust the chamber’s air fill level, ensuring an ideal fit. The design of these chambers required extensive research, testing, and teamwork to achieve the necessary functionality. The biggest challenge was obtaining certification as a medical device manufacturer and complying with the recently tightened EU regulations. This example highlights the importance of experience in the commercialization of medical technologies and knowledge transfer between academia and business. Most importantly, the medical product must address real patient needs.
It’s clear that experienced specialists play a crucial role in developing your solutions and technologies. What kind of expertise do your team members bring?
Our team comprises recognized figures from the medical field and skilled engineers whose diverse talents drive our projects’ innovation. Our engineers have extensive experience in designing and implementing advanced medical devices, while our medical experts provide valuable clinical insights essential for creating products that truly meet end-user needs. Collaboration between these groups is key to our success, as it allows us to create innovations that are not only technically viable but also have a real impact on improving or saving patients’ lives.
Do you believe Lublin has the potential to become a significant hub for medical technologies in cardiology and prosthetics?
Here in Lublin, we’ve developed unique, highly advanced solutions. Currently, we are pioneers in some technologies on a global scale. If our projects continue to develop as planned and we secure further funding for research, we have the potential to become a leading player in this field. The demand from doctors is significant, and since we offer innovative and advanced medical devices, we can compete technologically with industry leaders both in Poland and worldwide. I believe Lublin can become a major center for medical technology, and we are committed to making that a reality.
You mentioned high market demand. What does the demand look like for cardiology and prosthetics technologies in Poland and internationally?
Regarding limb prosthetics, the World Health Organization (WHO) estimates that about 40 million people worldwide are living with amputations (as of 2015). In Poland, approximately 30,000 amputations are performed annually, translating to a high rate of 7.4 per 100,000 inhabitants. For comparison, the rate in France is 4.3 per 100,000. It is estimated that soft silicone sockets could be a first-choice solution for at least half of the patients, about 14,000 annually.
In cardiology, cardiovascular diseases remain the leading cause of death in Poland. Each year, about 80,000 heart attacks occur in the country, with effective treatment mainly involving interventional cardiology procedures (e.g., coronary angioplasty with stent implantation, balloon coronary angioplasty, coronary angiography). Our diagnostic guidewire can effectively support interventional cardiologists during these procedures.
The global interventional cardiology market shrank by 20% due to COVID-19, reaching $11.1 billion in 2020. The market recovered in early 2022 and continues to grow steadily, projected to reach $16.2 billion by 2027.
What are your plans for the future development of the company?
Interventional medicine technologies are functional medical devices requiring production implementation for clinical trials. We are currently seeking investors and exploring European funding opportunities. To date, we have secured funding for each project under the Bridge Alfa program by NCBiR, totaling PLN 4,882,000. Our long-term plan is to commercialize our developed technologies to raise funds for further development of a modern R&D facility, allowing us to create new innovative solutions in response to needs identified by interventional doctors. The projects we are working on are just the beginning. To facilitate the production of our products for clinical trials, we will establish a manufacturing facility with enough capacity to fulfill contract orders and provide stable revenue. We have a very long-term vision.
In prosthetics, thanks to our engineering team’s expertise, we recently developed a proprietary device for automating the silicone processing used in prosthetic sockets. We already have international clients for this project, including one of the global market leaders. Our goal is to continue expanding into other foreign markets, with a focus on strengthening cooperation with Ukraine to support those affected by the war.






